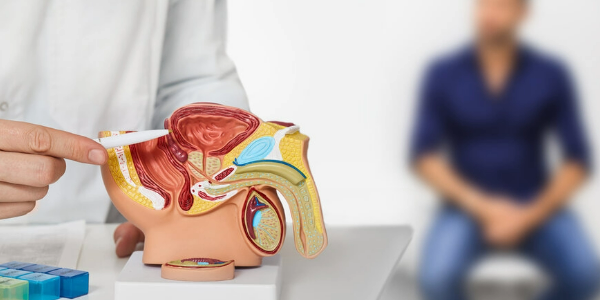
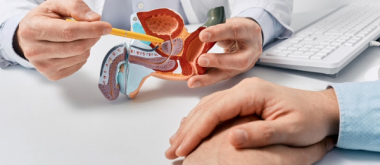
A large-scale international study led by researchers at UCL has found that combining two cancer drugs could significantly slow the progression of a severe and often fatal form of prostate cancer in men with certain genetic mutations. Published in Nature Medicine, the Phase III AMPLITUDE trial investigated whether adding niraparib, a targeted cancer therapy known as a PARP inhibitor1, could improve the effectiveness of the current standard treatment with abiraterone acetate and prednisone (AAP).
Targeting Genetic Weaknesses in Prostate Cancer
The study focused on men with advanced prostate cancer that had spread to other parts of the body and who were starting treatment for the first time. All participants had mutations in genes involved in homologous recombination repair (HRR), an important system that helps repair damaged DNA.
When these DNA repair genes do not function properly, cancer cells can multiply and spread more quickly. About one in four men with advanced prostate cancer at this stage have mutations in HRR-related genes, including BRCA1, BRCA2, CHEK2, and PALB2.
How the Study was Conducted
Currently, the standard treatment for advanced prostate cancer is AAP (or similar drugs). About one in five patients also receives chemotherapy with docetaxel. However, patients with HRR gene mutations typically experience faster disease progression and shorter survival times under standard treatment.
The AMPLITUDE study, led by Professor Gerhardt Attard of the UCL Cancer Institute, involved 696 men from 32 countries with an average age of 68. Half received the combination of niraparib and AAP, while the other half received standard AAP treatment with a placebo. More than half of the participants (55.6%) had mutations in BRCA1 or BRCA2. The study was double-blind, meaning that neither the patients nor their doctors knew who was receiving the active treatment.
Key Findings of the AMPLITUDE Study
After a median follow-up period of just over two and a half years (30.8 months), the researchers observed remarkable benefits of the combination of active substances:
- Lower risk of progression: Niraparib reduced the risk of cancer growth by 37% in all participants and by 48% in those with BRCA1 or BRCA2 mutations.
- Slower progression of symptoms: The time to symptom progression was approximately twice as long in those receiving niraparib. Only 16% of these patients showed significant symptom progression, compared with 34% in the placebo group.
- Potential survival benefit: There was a trend toward improved overall survival in the niraparib group, but longer follow-up is needed to confirm whether life expectancy is prolonged.
Expert Opinion
Professor Attard said: “Although current standard treatments are very effective for the majority of patients with advanced prostate cancer, a small but very significant proportion of patients derive only limited benefit from them. We now know that prostate cancer with alterations in HRR genes represents a significant group of patients in whom the disease recurs rapidly and takes an aggressive course. By combining it with niraparib, we can delay the recurrence of cancer and hopefully significantly extend life expectancy. These results are remarkable because they support comprehensive genomic testing at diagnosis and the use of targeted treatment for patients who can benefit most from it.
For cancers with a mutation in one of the eligible HRR genes for which niraparib is approved, a doctor should weigh the risks of side effects against the clear benefits of delaying disease growth and worsening symptoms.”
Side Effects and Safety
The treatment was generally well tolerated, but side effects occurred more frequently in the niraparib group. Significantly more cases of anemia and high blood pressure were reported with niraparib, and 25% of patients required blood transfusions. Treatment-related deaths were also higher in the niraparib group (14 versus 7), although the overall discontinuation rate remained low.
The study authors note that while the results are promising, further research is needed to confirm the long-term survival benefits and to investigate the impact of newer imaging techniques and more comprehensive genetic testing.
An estimated 1.5 million men worldwide are diagnosed with prostate cancer each year. In the UK, prostate cancer is the most common cancer in men, with more than 56,000 cases diagnosed each year and around 12,000 men dying from the disease annually.