A recent study reports that using reduced amounts of approved immunotherapeutics in malignant melanoma can lead to better tumor control while limiting side effects. The results come from researchers at the Karolinska Institutet and were published in the Journal of the National Cancer Institute. “The results are very interesting for oncology, as we show that a lower dose of an immunotherapeutic agent not only causes significantly fewer side effects, but also achieves better results in fighting tumors and longer survival,” says last author Hildur Helgadottir, a researcher at the Institute of Oncology-Pathology at the Karolinska Institute, who led the study.
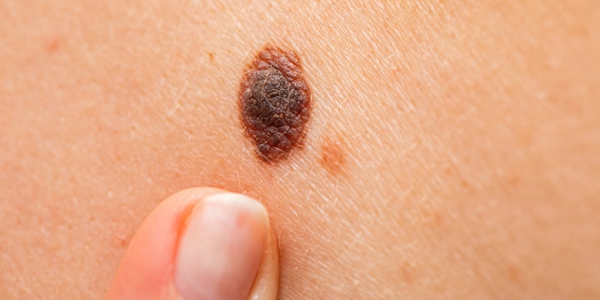
What are Malignant Melanomas?
Malignant melanomas are malignant tumors of the pigment-producing cells (melanocytes) of the skin. They are considered the most dangerous form of skin cancer because they grow rapidly and can metastasize to other organs at an early stage. Malignant melanomas often develop from previously inconspicuous moles, but can also occur spontaneously. The most important risk factors include intense or repeated UV radiation, sunburns—especially in childhood—fair skin types, and genetic predisposition. Early detection is crucial because melanomas are easily treatable in the early stages, while advanced melanomas have a significantly worse prognosis. Malignant melanomas can occur at any age, but are most common in middle-aged to older adults. Typically, the risk increases significantly from around the age of 40.
The treatment of malignant melanomas depends primarily on the stage of the disease. In the early stages, surgical removal of the tumor is the most important measure, whereby the melanoma is completely excised, often with a safety margin of healthy skin. For tumors that exceed a certain depth, a sentinel lymph node biopsy is also performed to check whether cancer cells have already spread to the lymph nodes. For advanced or metastatic melanomas, modern immunotherapies are used, such as checkpoint inhibitors, which activate the immune system to fight tumor cells. In addition, targeted therapies can be used if the melanoma has certain genetic changes, such as a BRAF mutation; in this case, BRAF and MEK inhibitors are used in combination. Less commonly, radiation therapy is used to relieve symptoms or in special cases, as well as classic chemotherapy.
The Advantages of Immunotherapy
Overall, while early-stage melanoma can usually be successfully treated with surgery, modern immunotherapy and targeted therapies have significantly improved survival rates for advanced disease in recent years. Immunotherapy for malignant melanoma offers numerous advantages over conventional treatment methods. It activates the body’s own immune system so that tumor cells can be specifically recognized and fought, while healthy cells are largely spared. As a result, the effect of the therapy can often continue beyond the treatment period, leading to long-lasting remissions that are rarely achieved with conventional chemotherapy. Immunotherapy has proven particularly effective in advanced or metastatic tumors, as it can stop or significantly slow tumor growth. In addition, immunotherapies can be combined well with other therapies, such as targeted BRAF and MEK inhibitors, to enhance treatment success.
Why Sweden is Pursuing a Modified Treatment Approach
The standard treatment for malignant melanoma in the context of immunotherapy is usually based on the approved dosages of nivolumab and ipilimumab. However, as the full-dose combination often leads to significant side effects, Swedish doctors are increasingly opting for a treatment regimen with a reduced amount of ipilimumab. This active ingredient is both the most expensive component of the treatment and the one most strongly associated with side effects. “In Sweden, we have more freedom in choosing the dosage for patients, while in many other countries, reimbursement guidelines dictate the dosages approved by the drug regulatory authorities,” says Hildur Helgadottir.
Improved Responses and Survival Rates With Reduced Ipilimumab Dose
Nearly 400 people with advanced, inoperable malignant melanoma participated in the study, which focused on this severe form of skin cancer. According to the results, patients who received the lower ipilimumab regimen showed stronger responses. A total of 49 percent of these patients responded to treatment, compared with 37 percent in the group receiving the conventional dose.
Progression-free survival, defined as the time a patient lives without disease progression, reached a median of nine months in the lower-dose group. In the conventional dose group, the median was three months. Overall survival also differed significantly, with medians of 42 months and 14 months, respectively.
Fewer Side Effects May Allow for Longer Treatment
Serious side effects occurred in 31 percent of those treated with the lower dose, compared with 51 percent of those who received the standard dose. “The new immunotherapies are very valuable and effective, but at the same time they can cause serious side effects that are sometimes life-threatening or chronic. Our results suggest that this lower dosage could allow more patients to continue treatment for a longer period of time, which is likely to contribute to better outcomes and longer survival,” says Hildur Helgadottir.
Although there were some differences between the treatment groups, the benefit of the lower dosage remained even after taking into account various factors, including age and tumor stage. As this was a retrospective observational study, the research cannot conclusively prove that the lower dose directly led to the improved outcomes.