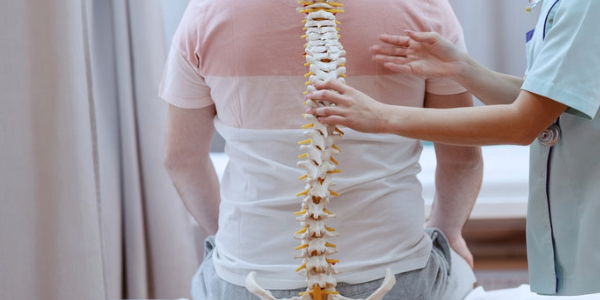
Researchers at Osaka Metropolitan University have developed a promising new method of treating spinal fractures using stem cells from adipose tissue, i.e., body fat. In animal experiments, this treatment successfully healed spinal injuries in rats that mimic osteoporosis-related fractures in humans. Since these cells are easy to extract even in older adults and place minimal stress on the body, this technique could be a gentle, non-invasive alternative for treating bone diseases.
Paralyzing Osteoporosis
Every year, a staggering 8.9 million fractures are caused by osteoporosis, which equates to one fracture every three seconds! The aging population is most susceptible to primary osteoporosis due to its frailty and often requires long-term therapy and support. Advances in healthcare and the accompanying increase in the aging population are straining available resources and underscoring the need for effective therapies for osteoporosis. Osteoporosis weakens bones, makes them brittle, and increases the risk of fracture.
Osteoporosis in menopause is mainly caused by a decline in the hormone estrogen, which previously had a protective effect on the bones. During the fertile years, estrogen supports both the formation and maintenance of bone mass by inhibiting its breakdown by osteoclasts. With the onset of menopause, estrogen levels drop sharply, causing bone breakdown to occur faster than bone formation, which makes bones thinner, more porous, and more susceptible to fractures. The vertebrae, wrist, and femoral neck are particularly affected, which significantly increases the risk of pain, vertebral fractures, or falls. Men are also affected with increasing age, although the decline in hormones in the stronger sex tends to be more gradual.
Over time, testosterone levels decline, and since testosterone directly and indirectly supports bone formation via estrogen production, bone mass gradually decreases. This process causes the bones to become more brittle and increases the risk of fractures, especially of the vertebrae, hips, and wrists. Men often only notice osteoporosis when a fracture occurs, as the disease does not cause any pain at first. Factors that further increase the risk include being underweight, lack of exercise, smoking, excessive alcohol consumption, calcium and vitamin D deficiency, and certain medications such as cortisone or anticonvulsants. Chronic diseases such as diabetes or chronic inflammation can also weaken the bones.
How Stem Cells Derived from Fat Help Rebuild Bones
Given the aging of the Japanese population, the number of people affected is expected to exceed 15 million. Among the various types of fractures caused by osteoporosis, compression fractures of the spine, known as osteoporotic vertebral fractures, are the most common. These injuries can lead to long-term disability and significantly impair quality of life, underscoring the need for safer and more effective treatments. Adipose-derived stem cells (ADSCs) show great potential for repairing bone damage. These multipotent cells can develop into various tissue types, including bone. When ADSCs are cultured into three-dimensional spherical clusters called spheroids, their ability to promote tissue repair increases. Pre-differentiating these spheroids into bone-forming cells further enhances their effectiveness in stimulating bone regeneration.
Led by Yuta Sawada, a student at the Graduate School of Medicine, and Dr. Shinji Takahashi, the Osaka research team used ADSCs to create bone-differentiated spheroids and combined them with β-tricalcium phosphate, a material commonly used in bone reconstruction. The mixture was applied to rats with spinal fractures, resulting in a significant improvement in bone healing and strength.
The researchers also observed that the genes responsible for bone formation and regeneration became more active after treatment, suggesting that this approach stimulates the body’s natural healing processes. “This study has demonstrated the potential of bone-differentiated spheroids using ADSCs for the development of new treatments for spinal fractures,” Sawada said. “Since the cells are derived from fat, the burden on the body is low, ensuring patient safety.” Dr. Takahashi added, “This simple and effective method can treat even difficult fractures and accelerate healing. It is expected that this technique will become a new treatment method that will help prolong the healthy lives of patients.”
Promising Prospects for Future Treatments
But there are other possible avenues. Earlier research from Japan has identified a promising agent for the treatment of osteoporosis. In fact, the induction of parathyroid hormone (PTH) signaling using the PTH-derived peptide teriparatide has shown a potent bone-promoting effect in patients with osteoporosis. This effect is mediated by osteogenesis, the process of bone formation involving the differentiation and maturation of bone-forming cells called osteoblasts. However, PTH induction is also associated with the differentiation of macrophages into osteoclasts, which are specialized cells responsible for bone resorption. Although bone remodeling by osteoblasts and osteoclasts is essential for maintaining skeletal health, PTH-induced osteoclast differentiation may reduce the effectiveness of treatment in patients with osteoporosis. However, the precise molecular mechanisms underlying the dual effects of PTH signaling in bone remodeling are not yet fully understood.
To fill this gap, Professor Tadayoshi Hayata and Ms. Chisato Sampei of Tokyo University of Science, together with their colleagues, conducted a series of experiments to identify drug-treatable target genes downstream of the PTH signaling pathway in osteoblasts. Corresponding author Prof. Hayata explains the rationale behind their study, published in the Journal of Cellular Physiology: “In Japan, an estimated 12.8 million people, or one in ten, suffer from osteoporosis, which can significantly impair their quality of life. Teriparatide is classified as a drug that promotes bone formation, but it also promotes bone resorption, which can limit bone formation. However, the full extent of its pharmacological effects is still unknown.”
New Target Molecule Discovered that Suppresses Bone Formation
The researchers treated cultured osteoblast cells from mice and mice with teriparatide. They then used advanced RNA sequencing analysis to evaluate PTH-induced changes in gene expression in both the cultured cells and bone cells isolated from the femurs of the treated animals. Among several upregulated genes, they identified a new PTH-induced gene, “Gprc5a,” which encodes an orphan G protein-coupled receptor that had previously been investigated as a therapeutic target. However, its exact role in osteoblast differentiation was not yet fully understood.
PTH induction is known to activate the cyclic adenosine monophosphate (cAMP) and protein kinase C (PKC) signaling pathways. Interestingly, the team found that, in addition to PTH induction, activation of cAMP and PKC also led to overexpression of Gprc5a, albeit to a lesser extent, highlighting the possible involvement of other molecular signaling pathways. Notably, the upregulation of Gprc5a was suppressed upon inhibition of transcription but remained unaffected upon suppression of protein synthesis, suggesting that Gprc5a may be transcribed early in response to PTH signals and serve as a direct target gene. In addition, the researchers investigated the effects of downregulation of Gprc5a on osteoblast proliferation and differentiation. Notably, PTH induction alone had no effect on cell proliferation, whereas downregulation of Gprc5a led to increased expression of genes associated with the cell cycle and markers of osteoblast differentiation. These results suggest that Gprc5a suppresses osteoblast proliferation and differentiation.
In their detailed investigation of the molecular mechanisms underlying the effects of Gprc5a in PTH-induced osteogenesis, the researchers identified activin receptor-like kinase 3 (ALK3) – a receptor of the bone morphogenetic protein (BMP) signaling pathway – as an interaction partner of Gprc5a. Consistent with their hypothesis, overexpression of Gprc5a indeed led to suppression of BMP signaling via receptors such as ALK3. Overall, these findings show that Gprc5a—a novel inducible target gene of PTH—negatively regulates osteoblast proliferation and differentiation by partially suppressing BMP signaling. Gprc5a can therefore be pursued as a novel therapeutic target in the development of treatments for osteoporosis. The study sheds light on the complex process of bone remodeling and explains the bone-promoting and bone-resorbing effects of PTH signaling. “Our study shows that Gprc5a could act as a negative feedback factor for the bone-forming effect of teriparatide. Suppressing Gprc5a function could therefore increase the efficacy of teriparatide in patients who do not respond to treatment. We hope that our research will lead to improved quality of life and healthy longevity for people with osteoporosis in the future,” concludes Prof. Hayata. These findings should pave the way for the development of effective therapies that can improve the lives of people with osteoporosis.
What Individuals Can Do to Minimize their Risk of Osteoporosis
To actively reduce the risk of osteoporosis, there are a number of things you can do in your everyday life without immediately resorting to medication. The key is to support bone metabolism, maintain bone density, and avoid falls. One of the most important measures is exercise. Strength training, weight-bearing activities such as walking, climbing stairs, or light jogging, as well as balance exercises. These not only strengthen the muscles, but also the bones, while reducing the risk of falls. Regular physical activity should be integrated into your daily routine as much as possible.
Nutrition also plays a central role. An adequate intake of calcium and vitamin D is crucial, as calcium supports bone formation and vitamin D facilitates the absorption of calcium in the intestines. Dairy products, green leafy vegetables, nuts, and fatty sea fish provide important building blocks. In addition, it is advisable to ensure a protein-rich diet, as protein is important for bone substance and muscle strength. A healthy lifestyle also contributes significantly. Smoking and excessive alcohol consumption accelerate bone loss and should be avoided if possible. Body weight also plays a role: underweight people have a higher risk, so being normal weight or slightly overweight is beneficial for bone health.
In addition, it is worth avoiding falls in everyday life, for example through good lighting, non-slip mats, and sturdy footwear. Regular check-ups such as DEXA scans can help detect changes in bone density at an early stage, so that targeted measures or therapies can be taken. This combination of exercise, nutrition, a healthy lifestyle, and prevention can significantly reduce the risk of osteoporosis.