The human eye is complex and irreparable, but structurally it resembles that of the freshwater apple snail, which can completely regenerate its eyes. Alice Accorsi, assistant professor of molecular and cell biology at the University of California, Davis, is investigating how these snails regrow their eyes—with the ultimate goal of helping people with eye injuries regain their sight. In a new study published in Nature Communications, Accorsi shows that apple snails and human eyes share many anatomical and genetic features.
Apple Snails Have Eyes like Cameras and a Strong Ability to Regenerate
“Apple snails are extraordinary organisms,” Accorsi said. “They offer a unique opportunity to study the regeneration of complex sensory organs. Until now, we lacked a system to study the complete regeneration of the eye.” Her team also developed methods for editing the apple snail’s genome, which they can use to study the genetic and molecular mechanisms of eye regeneration.
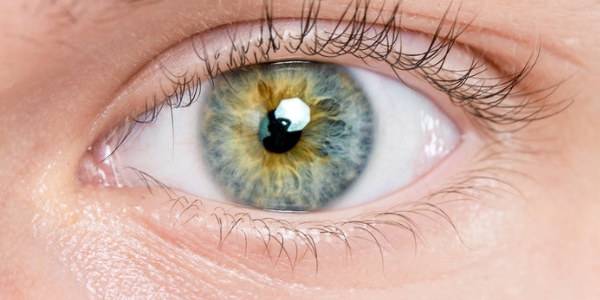
The golden apple snail (Pomacea canaliculata) is a freshwater snail species from South America. It is now invasive in many places around the world, but according to Accorsi, the very characteristics that make apple snails so invasive are also the reason why they are so well suited for laboratory experiments. Apple snails are resilient, their generation time is very short, and they have many offspring. In addition, apple snails are easy to breed in the laboratory and have “camera-like” eyes – just like humans. Snails have been known for centuries for their ability to regenerate. As early as 1766, a researcher discovered that decapitated garden snails can regrow their entire head. However, Accorsi is the first to use this property for regeneration research.
There are many types of eyes in the animal kingdom, but camera eyes are known for providing particularly high-resolution images. They consist of a protective cornea, a lens for focusing light, and a retina containing millions of light-sensitive photoreceptor cells. They are found in all vertebrates, some spiders, squids, and octopuses, as well as some snails. Using a combination of dissections, microscopy, and genome analysis, Accorsi’s team was able to show that apple snail eyes are anatomically and genetically similar to human eyes.
How to Regrow an Eye
So how do snails regrow their eyes after amputation? The researchers showed that the process takes about a month and involves several phases. First, the wound must heal to prevent infection and fluid loss, which usually takes about 24 hours. Then, unspecialized cells migrate to the area and multiply. Over the course of about a week and a half, these cells specialize and begin to form eye structures such as the lens and retina. On the 15th day after amputation, all structures of the eye are present, including the optic nerve, but these structures continue to mature and grow for several weeks. “We don’t yet have conclusive evidence that they can see images, but anatomically, they have all the components necessary to form an image,” Accorsi said. “It would be very interesting to develop a behavioral test to show that the snails can process stimuli with their new eyes in the same way as with their original eyes. We are currently working on this.”
The team also investigated which genes were active during the regeneration process. They showed that immediately after amputation, the snails had about 9,000 genes that were expressed at different levels compared to normal adult snail eyes. After 28 days, 1,175 genes were still expressed differently in the regenerated eye, suggesting that although the eyes appear fully developed after one month, complete maturation may take longer.
Genes for Regeneration
To better understand how genes regulate regeneration, Accorsi developed methods for editing the snail genome using CRISPR-Cas9. As a first test, the team used CRISPR/Cas9 to mutate a gene called pax6 in snail embryos. Pax6 is known to control the development and organization of the brain and eyes in humans, mice, and fruit flies. Like humans, snails have two copies of each gene – one from each parent. The researchers showed that apple snails with two non-functional versions of pax6 develop without eyes, proving that pax6 is also essential for initial eye development in apple snails.
Accorsi is now working on the next step: she wants to test whether pax6 also plays a role in eye regeneration. To determine this, the researchers must mutate or switch off pax6 in adult snails and then test their ability to regenerate. She is also investigating other eye genes, including genes that encode specific parts of the eye such as the lens or retina, as well as genes that control pax6.“If we find a set of genes that are important for eye regeneration and these genes are also present in vertebrates, we could theoretically activate them to enable eye regeneration in humans,” Accorsi said.